SERIOUS reactions linked to weight loss jab Mounjaro have increased by over 300 per cent in a single year.
Figures show 40,245 adverse reactions to tirzepatide, the active ingredient in the jab, were reported in the UK in 2025.
 Reports of adverse reactions to weight loss jab Mounjaro increased last yearCredit: Getty
Reports of adverse reactions to weight loss jab Mounjaro increased last yearCredit: Getty
The data came from the Medicines and Healthcare products Regulatory Agency’s (MHRA) Yellow Card Scheme – which allows patients, carers and medics to submit worries.
It comes after earlier this year that soaring numbers of slimmers have had emergency hospital treatment for suspected weight loss jab side-effects, with three deaths reported.
Mounjaro helps users lose weight by mimicking fullness hormones naturally released by the body and dulling hunger pangs.
It can it also help manage type 2 diabetes as it can have a positive effect on blood sugar.
MHRA stats show that 33,419 of the reactions reported were minor, whilst 6,755 were serious and 71 were fatal.
This is a near 340 per cent increase on the 9,153 adverse reactions reported in 2024 – 13 of which were fatal.
Overall, 32,075 reports were from women, 6,013 from men and 2,157 unknown.
Though the age of most patients reporting reactions is unknown, the age group with the most reports – 1,397 – was 30 to 39-year-olds.
The most commonly reported reaction in 2025 was ‘gastrointestinal disorders’, and nineteen of the 37,546 reports resulted in fatalities.
This was followed by ‘general disorders and administration site conditions’ (682) and nervous system disorders (670).
Reactions to other weight loss jabs like semaglutide – known as Wegovy or its diabetes counterpart Ozempic – were trending downwards, indicating more weight loss jabbers were opting for Mounjaro.
A total of 8,938 adverse reactions to semaglutide were reported to the Yellow Card scheme in 2024, including nine deaths.
In 2025, there were 7,146 fewer reports of adverse reactions but an increase in fatal outcomes, with 19 deaths linked to the jabs reported to the scheme.
The Yellow Card website states that “the information reported should not be interpreted as a list of possible side effects, nor should these data to be used to estimate the frequency of side effects or to compare the safety profile of different drugs”.
It follows reports that weight loss jabs could be triggering in .
Meanwhile, the MHRA last week , known medically as GLP-1 injections, could pose a risk of severe acute pancreatitis.
‘Don’t take it lightly’
For Karen Coe, 60, taking Mounjaro felt like she was being “ripped open by a knife”.
She said: “At first I had a headache and got dizzy.
“I had a few stomach cramps.
“On Monday it was excruciating. It was like being ripped open with a knife.”
Karen decided to try the injections to lose weight and help combat type 2 diabetes, and was prescribed them on the NHS.
But after her first injection on March 14, 2025, she began to get a headache and feel dizzy.
Three days later on March 17, she woke up at 5am with “excruciating” stomach pains, suffered with extreme diarrhoea and had to call for an ambulance.
She said: “I nearly passed out.
“I had to ask my husband to call for an ambulance.
“I was dizzy and really cold.
“They did my observations and said it was all ok.”
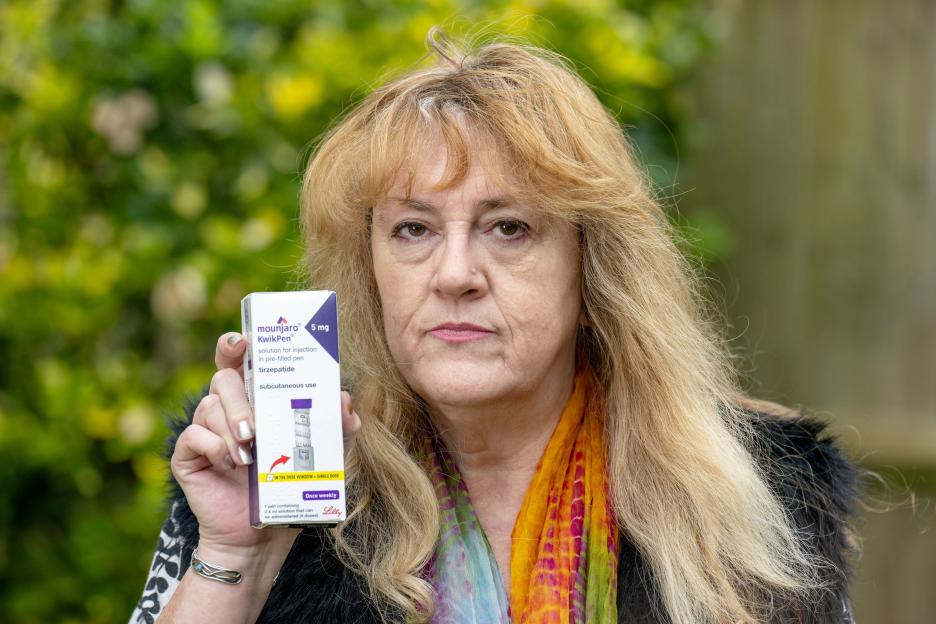 Karen Coe, 59, suffered an extreme reaction to single dose of the weight loss jab MounjaroCredit: SWNS
Karen Coe, 59, suffered an extreme reaction to single dose of the weight loss jab MounjaroCredit: SWNS
 She urged others to think carefully before taking the jabsCredit: SWNS
She urged others to think carefully before taking the jabsCredit: SWNS
Karen – who only injected herself once – was told to monitor her symptoms.
She spent the next 24 hours with stomach cramps and passing blood when she went to the toilet, she said.
Karen’s symptoms had eased off but when she had “massive blood clots” on March 24, 2025, she rushed back to A&E.
After an examination, she was referred on the urgent two week pathway to see a colorectal surgeon.
Doctors said her initial symptoms were likely caused by Mounjaro, she claims, but they haven’t confirmed the cause of her subsequent clotting complications.
The NHS lists nausea, diarrhoea, and abdominal cramps as potential side effects of the injection.
Karen was told to keep an eye on her symptoms and she was initially left weak and unable to eat.
What are the side effects of weight loss jabs?
Like any medication, weight loss jabs can have side effects.
Common side effects of GLP-1 injections include:
Nausea: This is the most commonly reported side effect, especially when first starting the medication. It often decreases over time as your body adjusts.
Vomiting: Can occur, often in conjunction with nausea.
Diarrhea: Some people experience gastrointestinal upset.
Constipation: Some individuals may also experience constipation.
Stomach pain or discomfort: Some people may experience abdominal pain or discomfort.
Reduced appetite: This is often a desired effect for people using Ozempic for weight loss.
Indigestion: Can cause a feeling of bloating or discomfort after eating.
Serious side effects can also include:
Pancreatitis: In rare cases, Ozempic may increase the risk of inflammation of the pancreas, known as pancreatitis, which can cause severe stomach pain, nausea, and vomiting.
Kidney problems: There have been reports of kidney issues, including kidney failure, though this is uncommon.
Thyroid tumors: There’s a potential increased risk of thyroid cancer, although this risk is based on animal studies. It is not confirmed in humans, but people with a history of thyroid cancer should avoid Ozempic.
Vision problems: Rapid changes in blood sugar levels may affect vision, and some people have reported blurry vision when taking Ozempic.
Hypoglycemia (low blood sugar): Especially if used with other medications like sulfonylureas or insulin.
She said: “Every few minutes I would go to the loo and pass blood.”
Karen won’t be continuing with her Mounjaro injections.
She said: “It can cause severe reactions and severe side effects.
“People should really think carefully and don’t take it lightly.”
Eli Lilly – who make Mounjaro, or tirzepatide – said: “Patient safety is Lilly’s top priority.
“We take reports regarding patient safety seriously and actively monitor, evaluate, and report safety information for all our medicines.
“Regulatory agencies conduct extensive independent assessments of the benefits and risks of every new medicine.
“Patients taking Mounjaro (tirzepatide), like with any other prescription medicine, may experience adverse events.
“Adverse events should be reported under the MHRA’s Yellow Card scheme.
“We encourage patients to consult their doctor or other healthcare professional regarding any side effects they may be experiencing and to ensure that they are getting genuine Lilly medicine.”